COVID-19 Vaccine Development

Simple. Powerful. Fast.
Immune System Monitoring with CyTOF
You need answers from every cell in each precious patient sample, and you need them quickly, reliably.
Mass cytometry, powered by CyTOF® technology, can expedite COVID-19 therapeutic and vaccine research studies with best-in-class, highly multiplexed, longitudinal immune monitoring capability.
Faster and more cost-efficient than single-cell RNA-seq, CITE-seq or AbSeq, mass cytometry also provides deeper resolution of immune cell phenotype and function than any flow cytometry or spectral cytometry system.
And CyTOF does it all in a single tube.
Webinar: Uncovering Immunological Mechanisms of Protection from Infection and Vaccination in Humans
In this presentation, Marcelo B. Sztein, MD, shows examples of his group’s use of mass cytometry to help advance the fields of vaccine development and pathogen-host interactions. He provides an in-depth look at his studies of Salmonella typhi (S. typhi), the causative agent of typhoid fever, an infectious disease of great public health importance.
Presenter:
 Marcelo B. Sztein, MD
Marcelo B. Sztein, MD
Center for Vaccine Development and Global Health
University of Maryland
Jump-start your SARS-CoV-2 vaccine and COVID-19 therapeutic research with the Maxpar Direct Immune Profiling System.
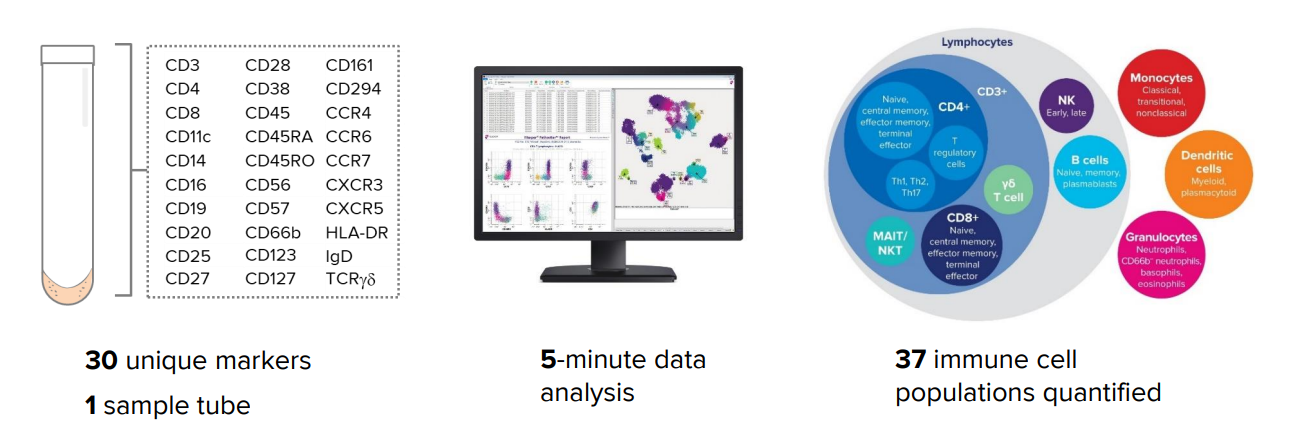
Best-in-class longitudinal immune profiling
- Systems-level panel reduces bias and helps you find the unknown.
- Contextual relationships between immune cell subsets maintained.
- Proven run-to-run reproducibility
Solves multi-site study challenges
- Simply freeze, store and ship samples.
- Easy-to-implement protocols and rapid, bias-free data analysis
- Demonstrated inter-site reproducibility
Panel flexibility and expansion
- 14-plus open channels for custom marker addition
- Maxpar Direct Expansion Panels provide flexible and focused solutions for further customization.
We chose the Maxpar Direct Immune Profiling Assay for this study* because it provides a robust and standardized solution for comprehensive immune monitoring that is technically easy to execute and harmonize across multiple study sites. ”
– Adeeb Rahman, PhD | Icahn School of Medicine at Mount Sinai
Related Webinars
Seminar: A Complete Immune Monitoring Solution with CyTOF; Ideal for Pandemics and Beyond
Frederik De Smet, MSc, PhD, presents early data from the COVID-19 Advanced Genetic and Immunologic Sampling (COntAGIouS) clinical research study (NCT04327570) using the Maxpar® Direct™ Immune Profiling Assay™ and Maxpar Pathsetter™ software.
Presenter:
 Professor Frederik De Smet, MSc, PhD
Professor Frederik De Smet, MSc, PhD
Assistant Professor
University of Leuven, Belgium
Webinar: Profiling of COVID-19 Patient Immune Responses
Ruth Montgomery, PhD, describes her team’s work using mass cytometry in infectious disease studies and how her team will contribute to the IMPACC study by analyzing endotracheal aspirates from participants.
Presenter:
 Ruth Montgomery, PhD
Ruth Montgomery, PhD
Professor of Internal Medicine
Director, Yale CyTOF Facility
Associate Dean for Scientific Affairs
Published research and trials using the Maxpar Direct Immune Profiling Assay
Publications
Publications Overviews
National Clinical Trials
Clinical trials using mass cytometry in vaccine research as of September 30, 2020.
Clinical Trial Name |
Sponsor |
Start Date |
Phase |
NCT Number |
|---|---|---|---|---|
| FluPRINT Study: Characterisation of the Immune and Transcriptional Response to LAIV | University of Oxford | October 2019 | NA* | 04222595 |
| CVD 38000: Study of Responses to Vaccination with Typhoid and/or Cholera | University of Maryland | November 2018 | 4 | 03705585 |
| High Dose Flu Vaccine in Treating Children Who Have Undergone Donor Stem Cell Transplant | Vanderbilt-Ingram Cancer Center/ National Cancer Institute | September 2016 | 2 | 02860039 |
| Investigating Enteric Fever—Salmonella Typhi and Paratyphi Challenge Study | University of Oxford, Oxford Vaccine Group | December 2014 | NA* | 02192008 |
| Genetic and Environmental Factors in the Response to Influenza Vaccination (SLVP028) | Stanford University | October 2014 | 1 | 03088904 |
| The Role of CD4+ Memory Phenotype, Memory, and Effector T Cells in Vaccination and Infection (SLVP030) | Stanford University | September 2014 | 1 | 03453801 |
| CVD 37000: Immunity and Microbiome Studies at Intestinal and Systemic Sites in Ty21a Vaccinated Adults | University of Maryland | October 2013 | 4 | 03970304 |
| Study of a New MVA Vaccine for Hepatitis C Virus | ReiThera Srl | December 2010 | 1 | 01296451 |
| Blood Donor CVD 5000 | University of Maryland | January 2004 | 4 | 03971669 |
Table 1. Clinical trials using mass cytometry to study vaccine response as of September 30, 2020. Source: clinicaltrials.gov * NA=Not applicable
Key Publications Applying Mass Cytometry for Vaccine Research
Publications
Get more information
Unless explicitly and expressly stated otherwise, all products are provided for Research Use Only, not for use in diagnostic procedures. Find more information here.
