Harness the power of mouse tumor models
How to enable studies that reveal significant details of tumor development, progression and treatment

Mouse tumor models are widely utilized in preclinical drug development and cancer studies, in which the ability to evaluate multiparametric response in the tumor microenvironment (TME) is crucial for predicting therapeutic efficacy. Mouse models allow us to detail the initiation of a disease process and its full progression in highly controlled settings, something we can’t necessarily study well in humans. This provides the opportunity to closely assess immunological and oncological processes for specific disease types that dictate tumor growth, metastasis and immune response, essential in identifying drug candidates for further clinical evaluation.
Imaging can enhance what we learn, putting different aspects of tumor growth, immune response and treatment outcomes into context.
Here’s a breakdown of four approaches to tissue imaging and what they can offer:
Looking at tissue architecture helps identify the underlying cellular and structural features of the tumors, unveiling spatial tissue coordinates for tumor cells, immune cells, vasculature, smooth muscle cells and components of the extracellular matrix (ECM). Since the architectural properties of tissues inform us about tumor progression, understanding how the microenvironment and surrounding areas are remodeled can provide insights into clinical diagnosis, prognosis and patient outcome.
In biomarker discovery and drug development, the tissue architecture can influence drug delivery into the tumor. Alterations and irregularities in the spatial arrangement of cells caused by tumor growth are important for monitoring treatment responses and detection of tumor recurrence and/or resistance.
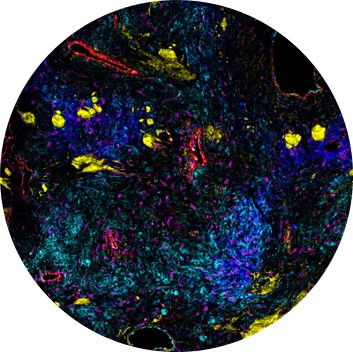
By creating a cellular atlas of the diseased mouse tissues, defining the heterogenous spatial landscape of the TME and assessing immune cell composition and positioning, multiplex imaging can provide insights into the biology of cancers and bolster understanding of fundamental and clinical cancer biology.
Gaining an understanding of dynamic biological systems and an in-depth look at cancer cell processes through imaging can assist in assessing activation state of cancer cells. These cellular processes include activation of canonical and non-canonical Ras signaling, metabolic activity, tumor growth and metastatic processes associated with epithelial-to-mesenchymal transition (EMT), which are all linked to promoting cancer morbidity by contributing to tumor initiation, invasion and resistance to therapeutic interventions.
Cancer is a dynamic disease: Tumors generally become more heterogeneous over time, and that heterogeneity helps create resistance to therapies. The TME often consists of a large number of diverse components, such as stromal fibroblasts, infiltrating immune cells, blood and lymphatic vessels, the ECM, cytokines and growth factors, that all play important roles in the development and progression of the tumor and have different levels of treatment sensitivity. Being able to accurately surveil and assess the many various subtypes cells in the TME can help deliver more effective and personalized therapies.
Phenotyping of key immune cells, including helper and cytotoxic T cells, B cells, macrophages and antigen-presenting cells, can help characterize specific immune cell populations and their spatial organization in the tumor immune microenvironment (TIME), the assessment of which is critical for discovering promising immunomodulatory cancer therapeutics.
The TME is thought to play a crucial role in the efficacy of immunotherapy and in mediating tumor immune evasion. Tumors can avoid or inhibit attacks from the immune system through mechanisms such as restricting antigen recognition, inducing T cell exhaustion, accumulating metabolites and signal factors in the TME or even limiting the nutrients available to immune cells. Since the TME has the potential to enhance the development and efficacy of immunotherapies, learning more about tumor-infiltrating cells and their spatial context using mouse preclinical cancer models is therefore crucial.
Through identifying the activation state of lymphoid and myeloid cells including immune cell proliferation and cytotoxic T cell activation, evaluating immune activation in and around the tumor can lead to a better understanding of disease mechanisms. It can also result in more effective identification of predictive factors that can shape a therapeutic approach, since understanding the role of innate immune cells in tumorigenesis and progression can improve therapeutic approaches.
Quickly and efficiently determining immune response is essential for evaluating immune function status, measuring the level of immune cells in the body, distinguishing immune responders and non-responders and ultimately advancing the success of cancer immunotherapies.
Imaging Mass Cytometry™ (IMC™) permits analysis of 40-plus distinct tissue and cellular markers simultaneously on tumor samples, providing a thorough evaluation of the spatial landscape of the TME.
The 28-plex ready-to-go Maxpar OnDemand™ Mouse Immuno-Oncology IMC Panel Kit (PN 9100005) consists of four modules that can be used separately or together and provides a standardized approach to developing a deep understanding of the tissue microenvironment in response to disease or therapy. Researchers can also further customize their panels by using open channels to easily add antibodies.
These four modular subpanels can be combined with the Maxpar® IMC Cell Segmentation Kit, which offers three individual plasma membrane markers for improved nucleus and plasma membrane demarcation, allowing for robust single-cell analysis.
Learn more about how you can easily characterize the breadth and depth of immune cell subpopulations to gain deeper insights into complex biology.
Check out other blog posts
Powerful, flexible tools to tackle public health challenges
Empower the generation of timely answers that can transform your research and position your laboratory as a center of excellence.
Deciphering one of the most challenging forms of cancer
A poster by Standard BioTools scientists sheds light on the spatial landscape of neoplastic brain tissue.
Unless explicitly and expressly stated otherwise, all products are provided for Research Use Only, not for use in diagnostic procedures. Find more information here.