Sample Identification
Sample identity and the integrity of genomic data are critical for execution of superior research. Particularly with complex multiphase studies, aliquots from a single specimen may be shared among researchers within a laboratory or with external collaborators and service providers.
It is a universally recognized priority to ensure accurate sample tracking and quality acquisition, all done through data reporting. This methodology can extend from high-throughput genomics centers to biorepositories or centralized cell line banks.
NEW FEATURED INSTRUMENT

X9™ High-Throughput Genomics System
Deep Insights With Nanoscale Genomics
The only genomics system for real-time PCR and NGS library preparation to support discovery through screening.

Confidently track samples and assess quality using automated microfluidics
Biobanks and genomics centers must overcome several challenges to ensure that they are providing the highest quality and the correct samples to research projects.
Sample mix-ups, contamination or handling errors can occur before or after samples enter the molecular laboratory or storage facility.
Implementing a standard genotyping workflow to confirm the identity and quality of each sample before analysis can maximize the integrity of study results.
Processing misidentified, poor-quality or contaminated samples may lead to incorrect interpretation of results.
The Advanta™ Sample ID Genotyping Panel provides a high-throughput and automated solution for molecular sample identification, enabling you to simultaneously perform molecular fingerprinting, quality assessment and integrity confirmation on every sample. The panel can be used with multiple genomic workflows and is customizable for any sample type.

Sample mix-ups, contamination or handling errors can occur before or after samples enter the molecular laboratory or storage facility.

Processing misidentified, poor-quality or contaminated samples may lead to incorrect interpretation of results.
molecular sample identification
What is molecular sample identification?

This proactive sample identification system is also known as sample ID or DNA fingerprinting. Panels of DNA markers are analyzed for each sample, generating a unique genotype or fingerprint. The genotype obtained is an indelible, nontransferable identifier for the sample.
Carefully selected markers can provide additional information, such as:
- Assessment of sample quality
- Genetic gender identity
- Population prediction
How is it used?

DNA samples can be fingerprinted (identified) prior to distribution.
Sample identity can be confirmed.
- Compare genetic sex to reported sex.
- Compare genotypes from distribution sample to accession genotypes.
Low-quality DNA issues can be identified.
- Low genotype calling rate
- Sample contamination

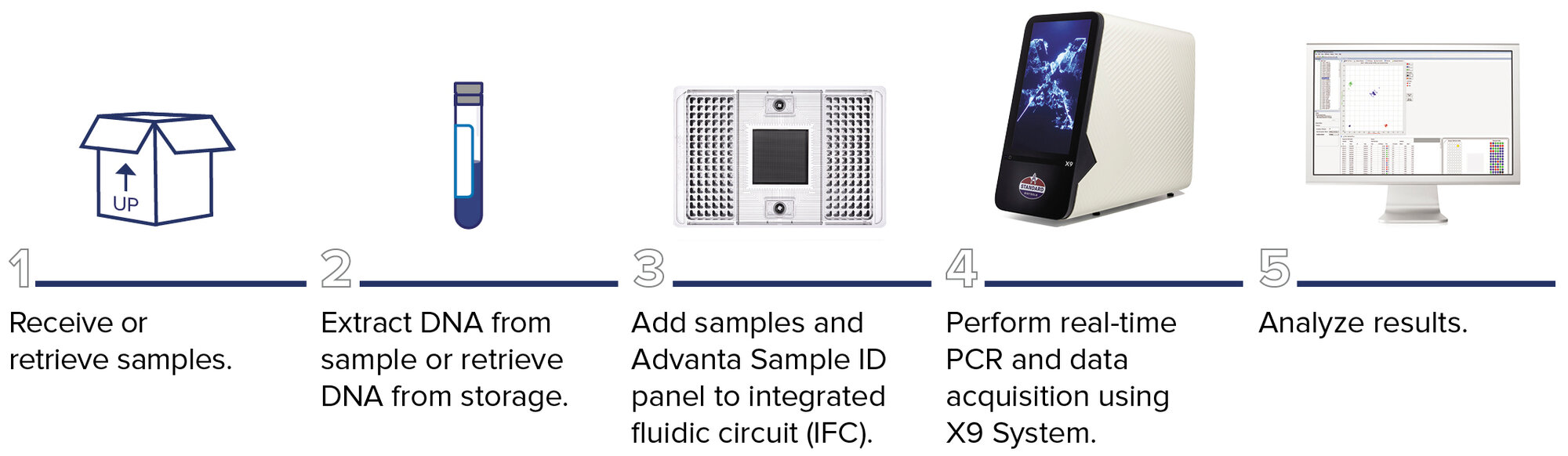
Advanta Sample ID workflow
Microfluidics-based fingerprinting provides outstanding reproducibility with automated reaction mix assembly, which enables high-throughput processing of up to 9,216 results per run. The nanoliter reaction volume requirements reduce reagent cost and maximize sample usage.
The workflow allows for:
- Maximum targets in individual reactions with no multiplexing
- Direct compatibility with common probe-based assays
- Flexibility with no pre-spotted assays
Webinar
Sample Tracking in Genomic Workflows Using the Biomark X System and New Analysis Pipeline Software
Speaker: Jordan Moore, Director, Field Applications, Standard BioTools™
Join us in this webinar to learn how automated nanoscale PCR reaction assembly and high-parameter singleplex target detection can be applied to simultaneously enable both sample traceability and sample integrity assessment in your facility's workflows. The presentation will describe our latest application for Biomark X™– the Advanta Sample ID Genotyping Panel, a 96-SNP genotyping assay that enables sample tracking via unique molecular fingerprinting – as well as quality assessment of genomic sample integrity. The webinar will highlight an ongoing collaboration with the DNA and Cell Bank at the Paris Brain Institute-ICM to develop a fast, safe and user-friendly software pipeline to store and analyze sample tracking and quality data from each sample.

Learn from the Expert

The Indiana University Genetics Biobank (IUGB) at the Indiana University School of Medicine is a high-throughput biobanking facility that supports numerous research programs. In this on-demand webinar, Kelly Nudelman describes the IUGB biobanking pipeline and how they have implemented sample tracking and quality checks in their workflows.

In this on-demand webinar, Jianhong Hu shares the details of how the Human Genome Sequencing Center (HGSC) at the Baylor College of Medicine has implemented SNP Trace™ as part of its NGS workflow. Then, in the same webinar, learn more about the Advanta Sample ID Genotyping Panel from Luke Stewart, Senior Field Applications Manager at Standard BioTools.

From high-throughput genomics centers to biorepositories or centralized cell line banks, ensuring accurate sample tracking and quality from acquisition through data reporting is a universally recognized priority.
During this webinar, we detail the Advanta Sample ID Genotyping Panel, a 96-SNP genotyping assay that enables sample identification and quality assessment. Leveraging microfluidics technology, the Advanta Sample ID workflow offers a number of benefits including decreased hands-on time and significant cost savings compared with traditional qPCR methods.

The DNA and cells bank at the Paris Brain Institute-ICM manages more than 50,000 samples and associated data and makes them available to the scientific community. In this on-demand webinar, Sylvie Forlani, Operational Manager of the ICM biobank, describes the implementation of the Advanta Sample ID Genotyping Panel in her facility’s workflow for sample tracking and quality check. She also introduces a fast, safe and user-friendly analysis pipeline her team developed to store and analyze each sample.

Samples used in biomedical research are often collected over years, in some cases from subjects who have died. Therefore, the value of these samples is priceless. Sample misidentification and mix-up are unfortunately common problems in biomedical research and can eventually result in the publication of incorrect data. In this on-demand webinar, Sara Tomei from Sidra Medicine compares the Fluidigm SNP Trace and the Agena iPLEX® Sample ID panels for the authentication of human genomic DNA samples.
customer stories
Learn more about how our customers are leveraging Standard BioTools technology
Kelly Nudelman, PhD

Kelly Nudelman and Tae-Hwi Schwantes-An discuss sample identification
Resources
Flyers & brochures
Posters
RELATED BLOG POSTS
-
Invest in Automation to Save Money in the Long Run
Where should managers put their focus to ensure their labs run smoothly? Learn about a new metric that can help improve lab efficiencies and cost savings for years to come.
-
Implementing microfluidics in any lab
No matter what laboratory you work in, using technology that streamlines your processes and saves resources is a crucial part of your success.
Unless explicitly and expressly stated otherwise, all products are provided for Research Use Only, not for use in diagnostic procedures. Find more information here.
